Amazing Healthcare Technology Advances of 2017
The referralMD Annual 2017 Healthcare Technology Review is here:
New year resolutions have been set with the goal of improving the future of healthcare with medical and dental technology advances.
- Will health systems accelerate the adoption of amazing technologies from the digital health startup community?
- Will we see new new drugs that cure major diseases?
- Will regulatory changes from the new Trump administration help or hurt?
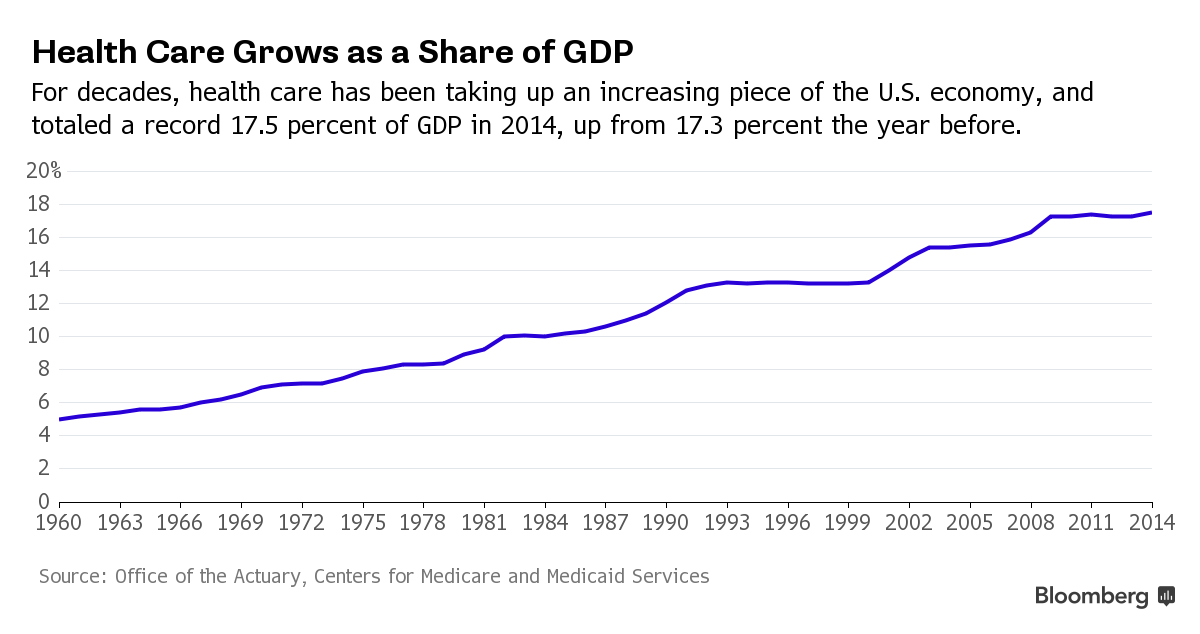
U.S. health care spending grew 5.8 percent in 2015, reaching $3.2 trillion or $9,990 per person. As a share of the nation’s Gross Domestic Product, health spending accounted for 17.8 percent. So what does this mean to all of our colleagues, friends, and families? It’s time to evaluate how we live take care of each other, or we could be in serious pain in the near future.
The referralMD healthcare technology review will showcase some amazing advances that could change the fabric of healthcare for the better and reduce the risk we would otherwise face.
Let’s get started
1. Healthcare Education: Augmented Reality
Education needs a revamp, and even more so healthcare education. Doctors are put through a gauntlet of requirements before they even see the light of day (AKA a paycheck) and this leads to supply issues. Currently it is estimated that the United States needs 96,000 more doctors just to meet the current needs.
So how does it get better?
Through robust 3D training tools offered by companies like 3D4Medical which helps doctors learn about Anatomy without having to cut open a cadaver.
Another interesting tool used by UCSF is called Echopixel.
It takes data from CT and MRI scans and transforms it into 3-D holographic images so she can view and interact with patient tissues and organs as if they were real physical objects. Medical 3-D imaging is not new, but the way organs appear to pop out of the screen and the ease at which the anatomy can be manipulated has never been seen before in medicine.
2. Clinical Patient Access Solutions
Nothing Is Certain But Death And Faxes
Believe it or not, healthcare still communicates with paper. Less than 10 percent of hospitals say they’ve been able to trade records entirely through their digital systems. Shocking right?
Patient access has always been a problem, only 54 percent of all faxed patient referrals result in scheduled appointments due to the complexities of communication between health systems, or the lack thereof.
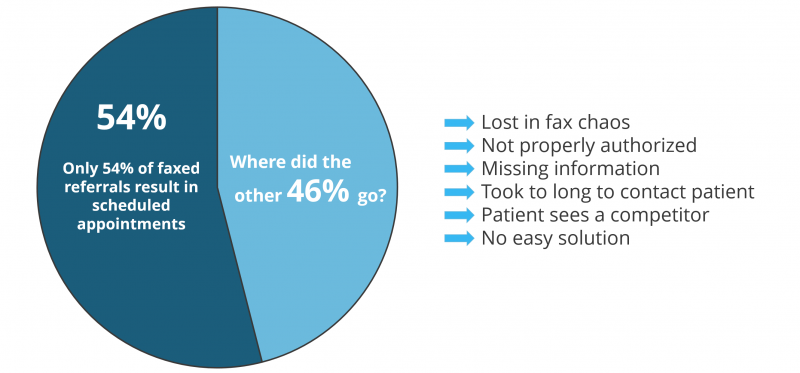
Ability to get a patient into an appointment is critical. Not only for a patient’s health, but for the viability of a health system from a revenue standpoint, its reputation, the insurance company, and even medical device and pharma companies. It is a tight knit ecosystem where if one chain in the communication link breaks down then all heck breaks lose and everyone suffers.
Great thing is there are finally platforms on the market that helps providers and staff route patients to the most appropriate high quality clinician in a community using SmartMATCH Technology from referralMD. Best of all it can integrate into the any EMR systems on the market like Epic, Cerner, eClinicalWorks, and Allscripts while reducing referral leakage saving health systems 100’s of millions.
ReferralMD Analytics Dashboard showing real-time leakage numbers
3. Human Head Transplants
Sergio Canavero , an Italian neurosurgeon, intends to attempt the first human head transplant by 2017, though no successful animal transplants with long-term survival have yet been made. Because of the difficulty of connecting the spinal cord, Canavero has suggested improvements in the process using a special blade and polyethylene glycol, a polymer used in medicine as well as in everything from skin cream to the conservation of the Mary Rose, can help start growth in spinal cord nerves.
Being able to surgically remove the head in an orderly fashion should allow surgeons to then reattach all the nerves and blood vessels to the new body, once that pesky donor head is removed. A special bio-compatible glue will hold the spinal cord together so it can fuse with the donor body. The patient will then be put in a drug-induced coma for four weeks while the connection between the head and body heals. It’s the reattachment process that’s the most unlikely part of all this. There’s never been a successful procedure that reattached a fully severed primate spinal cord.
Canavero says all the technology he needs is available, and estimates the procedure will take about 36 hours and require the services of 150 medical professionals. He expects a 90% chance of success, as in a 90% chance the patient is up and walking around a few months after the surgery. This all still sounds like science fiction, and medical professionals are mostly skeptical of Canavero’s plan. He seems set to try, though. And who knows? Maybe it’ll work. A few years ago face transplants seemed like science fiction. Even if this does work, the process will be obscenely expensive. Plus, it will give an entire body full of transplantable organs to a single person. It’s unclear if this would be considered ethical when there are so many people waiting for transplants.

Valery Spiridonov, a man who has volunteered to be the first person to undergo a head transplant, attends a news conference in Vladimir, Russia, June 25, 2015. The 30-year-old Russian, who has a degenerative muscle condition known as Werdnig-Hoffman, wants to become the first person ever to undergo a human head transplant. REUTERS/Maxim Zmeyev TPX IMAGES OF THE DAY – RTR4YX94
4. Qualcomm Tricorder 2017 Update
Diagnosing patients has always been challenging without in-depth testing, one day soon we may have devices that can help determine aliments in seconds versus hours or days. The future is coming and Star Trek fans like me are hoping it’s sooner then later.
Last year we reported on the Qualcomm Tricorder Xprize. The finalists have been chosen and narrowed down to 2 teams. Dynamical Biomarkers Group and Final Frontier Devices. Congrats everyone you earned it!
5. Heart in a Box: Warm Blood Perfusion System
Cardiac transplantation, also called heart transplantation, has evolved into the treatment of choice for many people with severe heart failure who have severe symptoms despite maximum medical therapy. Survival among cardiac transplant recipients has improved as a result of improvements in treatments that suppress the immune system and prevent infection.
Unfortunately, the number of heart donors has reached a plateau despite an increasing number of potential recipients. More than 5000 cardiac transplants occur each year around the world, although it is estimated that up to 50,000 people are candidates for transplantation [1]. This critical organ shortage means that healthcare providers must strictly evaluate who should receive a heart transplant.
For many years, surgeons in the United States have preserved organs in a cold solution and transported in coolers to the the receiving patient in need. But this process can cause damage to the heart and in some cases render it unusable.
So what is different?
A new process, called warm perfusion, can keep hearts beating and lungs “breathing” while allowing doctors to assess and treat organs to make them last longer, studies suggest.
6. Leadless Pacemakers
Pacemakers have come along way since the 60s, where investor Dr. Wilson Greatbatch accidentally invented it. The story goes that Greatbatch, working for a doctor at the Chronic Disease Research Institute, was designing a circuit to help record fast heart sounds. By mistake, he grabbed the wrong resistor from a box and plugged it into the circuit he was making. The circuit pulsed for 1.8 milliseconds and then stopped for 1 second and then repeated. Greatbatch recognized the lub-dub rhythm.
“I stared at the thing in disbelief,” he said. This was exactly what was needed to drive a sick human heart! For the next five years, most of the world’s pacemakers used that simple blocking oscillator design — just because of Greatbatch’s accident.
Fast-forward to today. Companies like Medtronic and St. Jude Medical have created versions called Micra (Medtronic) and Nanostim (St. Jude) that reduce potential risks of transvenous pacemakers such as serious infection.
7. Advanced Immunotherapies to Treat Cancer
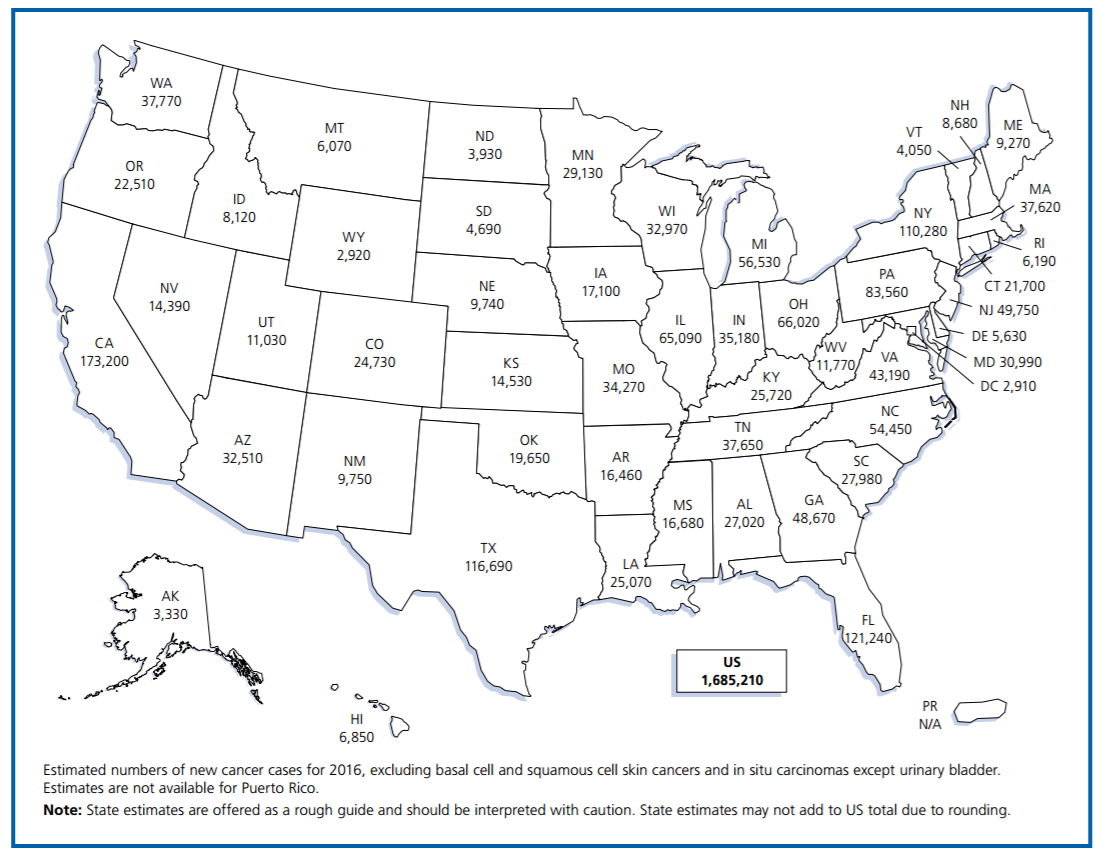
According to the American Cancer Society, over 1.5M new cases of cancer occurred in 2016. But new treatment options could help dramatically in the near future.
Scientists at Juno Therapeutics reported at the American Society of Hematology (ASH) meeting that, in an ongoing Phase 1 trial, its chimeric antigen receptor (CAR) T-cell therapy, JCAR015, put 24 of 27 adults with refractive acute lymphoblastic leukemia (ALL) into remission, with six patients remaining disease free for more than a year (ASH 2014, Abstract 382, 2014).
This disease is extremely hard to treat and progresses rapidly when it becomes refractory; most patients die within a few months. “This response rate is unprecedented for patients who had stopped responding to all other treatments,” says Michel Sadelain, a founding director of Memorial Sloan Kettering’s Center for Cell Engineering and a co-founder of Juno.
How does it work?
The use of human T cells as therapeutics to re-engage the immune system has the potential to revolutionize the way cancer is treated. Juno’s technologies genetically engineer a patient’s own T cells to recognize and kill cancer cells. These cellular therapies have the potential to be effective regardless of the type of previous treatments patients have experienced and may avoid the long-term side effects associated with current treatments. We use two different technologies to target cancer cells and activate T cells, CARs and TCRs.
8. In Silico Clinical Trials (Organs on a chip)
Clinical trials in their current form take many years to complete and testing a single compound can cost more then $2B due the complexities of our FDA.
The process is long and tedious prior to approval of any new drug by regulatory agencies. Idea is to ensure that drug should show it’s effectiveness in a convincing manner and it’s effects should weigh over it’s side effects before it reaches market.
Process of drug development involves various stages:
• in Vitro/Cell-based studies
• in Vivo/Animal Model studies
• Phase 1 trials
• Phase 2 trials
• Phase 3 trials
• Approval
• Phase 4 trials
Each of these steps requires the company to:
- pay to write the clinical trial protocol,
- pay to get the IRB approval,
- pay doctors to treat patients with your experimental formulation,
- pay to have statisticians set up randomization and blinding,
- pay to recruit patients to the trial,
- pay clinical trial coordinators at each clinical site to counsel the patients, monitor the treatment, record results,
- pay any toxicity and imaging work (i.e., x-rays) related to the trial,
- pay the patients to come back at each follow-up date,
- pay the regulatory folks to audit clinical trial sites,
- pay for statistical analysis and regulatory submission, etc.
Researches are now starting to experiment with new technologies that will help reduce the time and cost of such trials that kill millions of animals every year.
Wyss Institute researchers and a multidisciplinary team of collaborators have engineered microchips that recapitulate the microarchitecture and functions of living human organs, including the lung, intestine, kidney, skin, bone marrow and blood-brain barrier. These microchips, called ‘organs-on-chips’, offer a potential alternative to traditional animal testing. Each individual organ-on-chip is composed of a clear flexible polymer about the size of a computer memory stick that contains hollow microfluidic channels lined by living human cells interfaced with a human endothelial cell-lined artificial vasculature, and mechanical forces can be applied to mimic the physical microenvironment of living organs, including breathing motions in lung and peristalsis-like deformations in the intestine. Because the microdevices are translucent, they provide a window into the inner workings of human organs.
Short video explaining the technology.
9. 3D Printed Drugs
3D printing has been around for many years; predominantly been used in manufacturing. This type of printing, also called stereolithography, can create almost any object by fusing different materials, layer by layer, to form a physical version of a digital 3D image. Over the past 15 years, 3D printing has expanded into the healthcare industry, where it’s used to create custom prosthetics and dental implants. Now, there may be an opportunity to use it for personalized healthcare as well.
Here are three other ways 3D printing could change the pharmaceutical world forever.
1. Personalized drug dosing
3D printing could add a whole new dimension of possibilities to personalized medicine. In its most simplistic form, the idea of experts and researchers is to produce personalized 3D printed oral tablets. Medical writer C. Lee Ventola has conducted extensive research on this for her publication, “Medical Applications for 3D Printing: Current and Projected Uses.” She writes that personalized, 3D-printed medications may serve particularly well for patients who respond to the same drugs in different ways.
Below is the first 3D drug printed by Aprecia Pharmaceuticals
Additionally, a doctor or a pharmacist would be able to use each patient’s individual information — such as age, race and gender — to produce their optimal medication dose, rather than relying on a standard set of dosages. 3D printing may also allow pills to be printed in a complex construct of layers, using a combination of drugs to treat multiple ailments at once. The idea is to give patients one single pill that offers treatment for everything they need.
2. Unique dosage forms
3D printing could also be used to create unique dosage forms in the pharmaceutical production process. In the process, the idea would be to use inkjet-based 3D printing technology to create limitless dosage forms. According to experts, it is likely that this could challenge conventional drug fabrication. The process to create novel dosage forms has already been tested for many drugs, and we will only witness more innovation as time moves on.
3. More complex drug release profiles
Drug release profiles explain how a drug is broken down when taken by the patient. Designing and printing drugs firsthand makes it much easier to understand their release profiles. 3D printing makes it possible to print personalized drugs that facilitate targeted and controlled drug release by printing a binder onto a matrix powder bed in layers. This creates a barrier between the active ingredients, allowing researchers to study the variations of the release more closely. As drug manufacturers start to understand the full set of opportunities allowing them to make more effective drugs, there will likely be more research and investment into this area in the coming years.
10. Synthetic Hormones for your Heart
Heart disease still reigns as the leading cause of death in America, surpassing cancer by a small margin.
- Heart disease: 614,348
• Cancer: 591,699
• Chronic lower respiratory diseases: 147,101
• Accidents (unintentional injuries): 136,053
• Stroke (cerebrovascular diseases): 133,103
• Alzheimer’s disease: 93,541
• Diabetes: 76,488
• Influenza and pneumonia: 55,227
• Nephritis, nephrotic syndrome, and nephrosis: 48,146
• Intentional self-harm (suicide): 42,773
Around 1 in 4 people who are hospitalized for heart failure don’t last much longer than a year.
But a new drug called Serelaxin has upped the odds of survival by as much as 37 percent, according to a University of California, San Francisco study. It’s a synthetic version of the hormone relaxin, which is produced by pregnant women to help with the increased stress carrying a fetus places on the heart.
Although Novartis, the maker of Serelaxin, coined RLX030 and by the trade name Reasanz, failed FDA approval, it will still remain in development pending modifications requested by the FDA.
1. One was Novartis’ reliance on one clinical trial to assess serelaxin’s effect on dyspnea rather than at least two, or one trial with data for multiple studies.
2. Another concern raised by Blank and McDowell was the single trial’s focus on serelaxin’s beneficial effect on dyspnea, dismissed by FDA as an exploratory finding that did not address the AHF indication of the experimental drug
3. The FDA staffers also questioned whether the trial’s primary endpoint would have been achieved if it hadn’t assigned the worst reported score to patients who died or had worsening heart failure during the first five days.
11. Young Blood Antiaging – Fountain of Youth?
Is the fountain of youth becoming a reality? A new treatment option maybe in our future in which blood of younger people under 25 could be used to reverse the effects of aging.
Companies like Alkahest are developing trials to identify the key proteins in plasma that rejuvenate or age human tissues and then manufacture a product that uses them – could take 10 to 15 years. In the near term, the company has another strategy. Earlier this year, the Spanish blood products firm, Grifols, pledged $37.5m for a 45% stake in Alkahest. With another $12.5m, the company will bankroll more research in exchange for rights to Alkahest’s first products. Over the next two years, Alkahest will take human plasma and divide it into fractions that are rich in different proteins. Each fraction will then be tested in mice to see if they boost brain function. Any that do will be swiftly introduced into human trials and developed into the first generation of products.
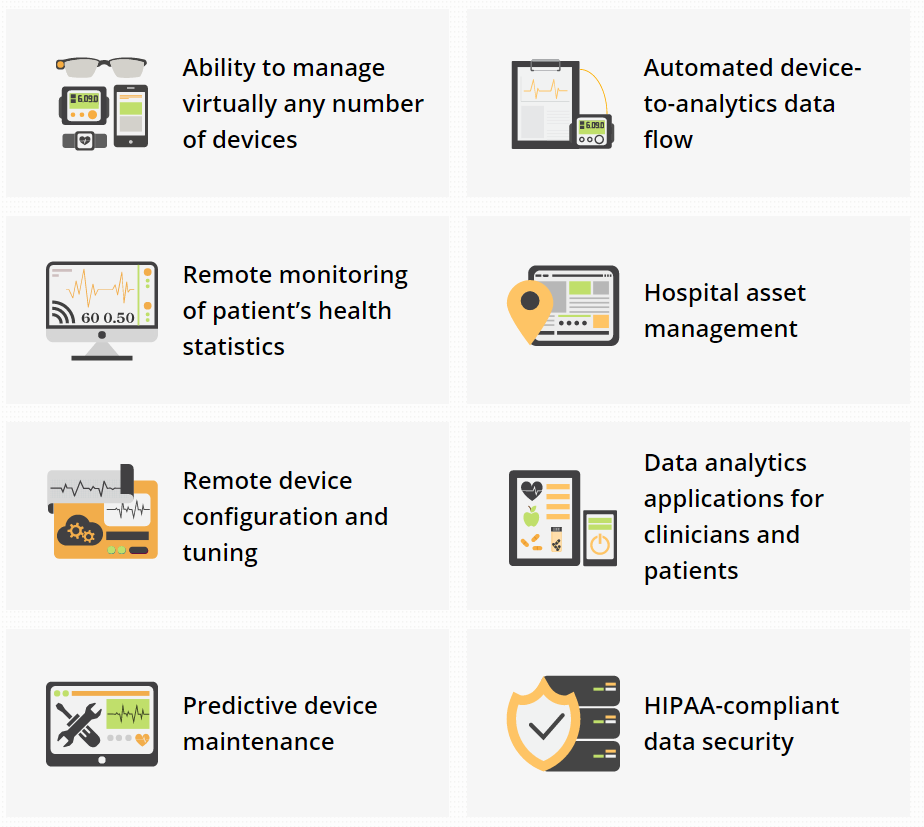
12. Internet of Things for Healthcare (IOT)
The Internet of Things is a hot topic now a days, or IoT which is the inter-networking of physical devices, vehicles (also referred to as “connected devices” and “smart devices”), buildings, and other items—embedded with electronics, software, sensors, actuators, and network connectivity that enable these objects to collect and exchange data.
Senior healthcare solutions director at Stanley Healthcare, Joel Cook, described the ways in which many of Stanley’s customers use IoT in healthcare. For example, hospitals take advantage of the technology for real-time location serviceswith badges that can track patients, staff and medical devices. “Many of our customers are using this equipment for asset management,” Cook said. Such assets include infusion pumps, wheelchairs, defibrillators, scales and other items that employees tend to tuck into out-of-sight corners yet are needed frequently for treating patients.
With Internet of Things devices, clinicians “in the PACU … can see what’s going on in the ORs, where they are in the case, and can therefore interpret when people are going to arrive in PACU,” Cook explained. “And likewise, people up on the med-surg floor can see what’s going on in PACU” and prepare for new patient arrivals.
In addition to real-time location services, Stanley’s IoT devices also help with environmental monitoring — for example, checking the temperatures of refrigerators or IT closets — and hand hygiene compliance.
Companies like the KAA project have created a open-source IOT platform which allows OEMs and healthcare system integrators to establish cross-device connectivity and implement smart features into medical devices and related software systems.
Healthcare companies can integrate Kaa’s functionality into their products were able to achieve IoT goals faster and at little expense.
13. Consumerism in Healthcare
Consumerism has been making inroads into the healthcare industry for at least a decade, with patients increasingly acting like consumers who have a choice in their healthcare options, trying to make the best decisions for quality and cost just as they do with any other commodity. The trend has been accelerated by the Patient Protection and Affordable Care Act, which left many consumers with large deductibles that put more pressure on them to find the most cost-effective care for the dollars coming out of their own pockets.
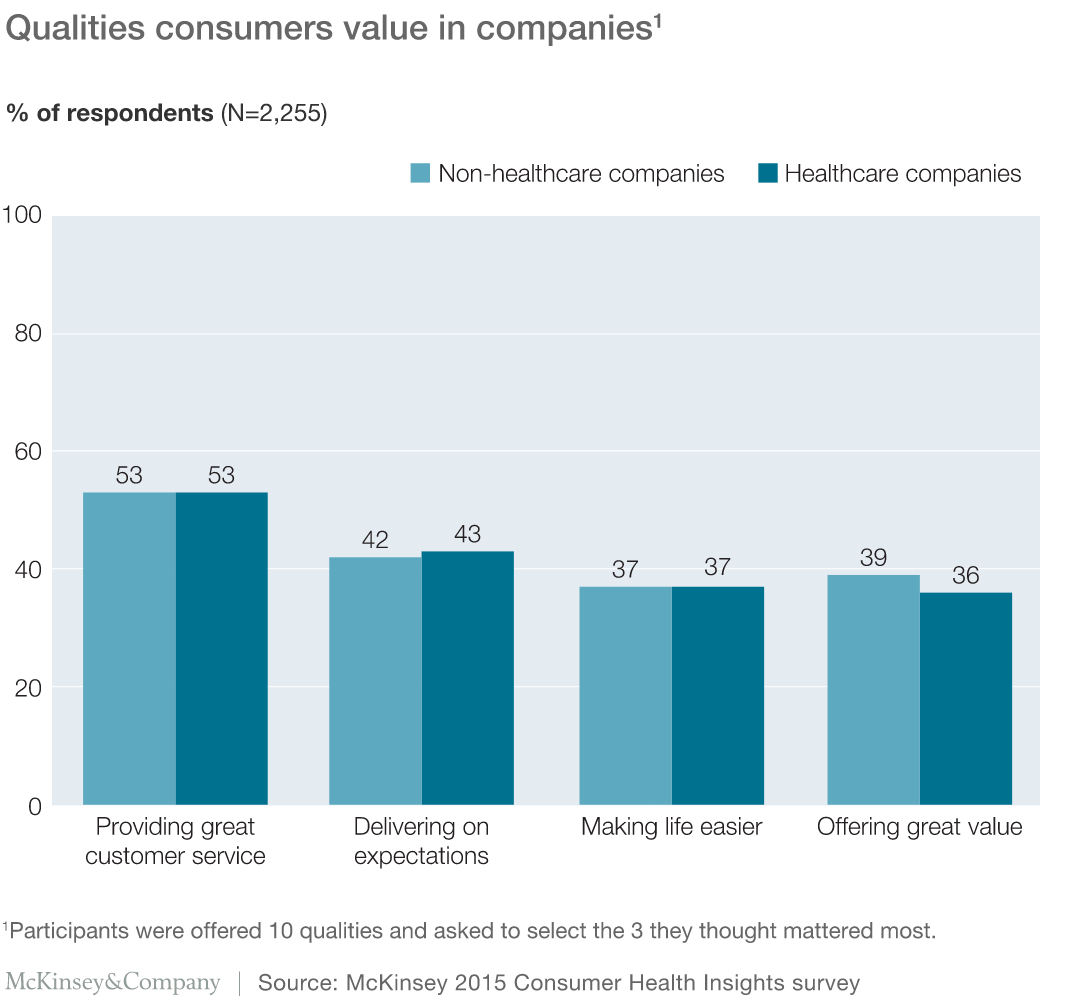
A recent study by McKinsey&Company sited more than half the participants cited great customer service as important for non-healthcare and healthcare companies alike. Other qualities that the participants identified as important for both sets of companies were delivering on expectations, making life easier, and offering great value.
Consumerism appears to have more impact on outpatient services than inpatient care, says Mark Bogen, senior vice president of finance and chief financial officer at South Nassau Communities Hospital, a 400-staffed-bed acute care facility in Oceanside, New York. Patients still rely on their physicians to refer them to the appropriate hospital and generally don’t question that choice unless the facility is out of network. It’s only at that point that most patients will speak up and ask about an in-network alternative, he says.
South Nassau’s Bogen expects to see the consumerism movement grow in the outpatient sector but says he hopes it doesn’t achieve any more presence in inpatient care than it already has.
“I still believe it’s crass to call them consumers,” he says. “On the outpatient side, it’s more fitting that people will shop for price, and you could argue that much of the outpatient services are commodities, but I don’t believe that to be true from an inpatient perspective, and I would hate to have people make what ultimately could be a life-and-death decision on the basis of cost.”
Still, Herzog says he sees consumerism as a tide that won’t be turned back, so he advises healthcare organizations to accept the change and respond accordingly.
Companies like referralMD, Healthgrades, and Vitals offer patients and providers tools to gauge which providers are both cost effective and high quality. Only time will tell as more data is available to showcase to consumers which providers are “Best” within a market place.
14. BioElectronics
Imagine having the equivalents of a gatekeeper, a multilingual translator, an air traffic controller, and a bouncer in your nervous system. Now imagine that this team can modulate and redirect signals from the brain and the rest of your nervous system to other systems and organs in your body. Any signals heading the wrong way or behaving erratically could be course-corrected along the way. That’s a rough translation of the concept of bioelectronics.
GSK’s Bioelectronics R&D unit is pursuing a relatively new scientific field that could one day result in a new class of medicines that would not be pills or injections but miniaturised, implantable devices. GSK believes that these devices could be programmed to read and correct the electrical signals that pass along the nerves of the body, including irregular or altered impulses that can occur in association with a broad range of diseases. The hope is that through these devices, disorders as diverse as inflammatory bowel disease, arthritis, asthma, hypertension and diabetes could be treated.
If Dr Daniel Chew’s work goes to plan, diabetics and sufferers from a host of other diseases may one day no longer need to inject themselves or take pills. He’s working to develop a tiny implant that could read and alter electrical signals that pass along nerves in the body and help control illness.
“Our work has huge potential to treat a vast array of different diseases,” says Chew. “Five years ago, bioelectronic medicine was barely even a concept.” Before he joined GSK, Chew spent a decade in research, which included investigating the use of electronic implants to treat patients with spinal cord injuries and help restore bladder and bowel control. “My work was pre-clinical in the lab but I also worked closely with vets to apply these electronic implants to treat pet dogs that had suffered similar trauma,” he explains.
15. Cognitive Computers
The amount of work we are tasked at performing is ever increasing, yet we are asked to do it with less resources then ever before. In the future as costs come down more health systems will be able to leverage advanced computer systems to help provide clinical support for their patients.
Currently the volume of healthcare data recently reached 150 exabytes. At projected growth rates, the volume of healthcare data will soon be zettabyte and yottabyte scale. That’s enough data to fill a stack of DVDs that would stretch from Earth to Mars.
Here is a chart that explains what those numbers mean:
Gigabyte (1 000 000 000 Bytes)
- 1 Gigabyte: A pickup truck filled with paper OR A symphony in high-fidelity sound OR A movie at TV quality, Surfing the Internet and watching videos = 100 MB/hr for 10 hours.
- 2 Gigabytes: 20 meters of shelved books OR A stack of 9-track tapes
- 20 Gigabytes: A good collection of the works of Beethoven OR 5 Exabyte tapes OR A VHS tape used for digital data
- 50 Gigabytes: A floor of books OR Hundreds of 9-track tapes
- 100 Gigabytes: A floor of academic journals OR A large ID-1 digital tape
- 200 Gigabytes: 50 Exabyte tapes
Terabyte (1 000 000 000 000 Bytes)
- 1 Terabyte: X-ray films in a large technological hospital OR 50000 trees made into paper and printed OR Daily rate of EOS data (1998)
- 2 Terabytes: An academic research library OR A cabinet full of Exabyte tapes
- 10 Terabytes: The printed collection of the US Library of Congress, or one human brain
- 50 Terabytes: The contents of a large Mass Storage System
Petabyte (1 000 000 000 000 000 Bytes)
- 1 Petabyte: 5 years of EOS data (at 46 mbps)
- 2 Petabytes: All US academic research libraries
- 20 Petabytes: Production of hard-disk drives in 1995
- 200 Petabytes: All printed material OR Production of digital magnetic tape in 1995
Exabyte (1 000 000 000 000 000 000 Bytes)
- 5 Exabytes: All words ever spoken by human beings.
- From wikipedia:
- The world’s technological capacity to store information grew from 2.6 (optimally compressed) exabytes in 1986 to 15.8 in 1993, over 54.5 in 2000, and to 295 (optimally compressed) exabytes in 2007. This is equivalent to less than one 730-MB CD-ROM per person in 1986 (539 MB per person), roughly 4 CD-ROM per person of 1993, 12 CD-ROM per person in the year 2000, and almost 61 CD-ROM per person in 2007. Piling up the imagined 404 billion CD-ROM from 2007 would create a stack from the earth to the moon and a quarter of this distance beyond (with 1.2 mm thickness per CD).
- The world’s technological capacity to receive information through one-way broadcast networks was 432 exabytes of (optimally compressed) information in 1986, 715 (optimally compressed) exabytes in 1993, 1,200 (optimally compressed) exabytes in 2000, and 1,900 in 2007.
- According to the CSIRO, in the next decade, astronomers expect to be processing 10 petabytes of data every hour from the Square Kilometre Array (SKA) telescope.[11] The array is thus expected to generate approximately one exabyte every four days of operation. According to IBM, the new SKA telescope initiative will generate over an exabyte of data every day. IBM is designing hardware to process this information.
Zettabyte (1 000 000 000 000 000 000 000 Bytes)
- From wikipedia:
- The world’s technological capacity to receive information through one-way broadcast networks was 0.432 zettabytes of (optimally compressed) information in 1986, 0.715 in 1993, 1.2 in 2000, and 1.9 (optimally compressed) zettabytes in 2007 (this is the informational equivalent to every person on earth receiving 174 newspapers per day).[9][10]
- According to International Data Corporation, the total amount of global data is expected to grow to 2.7 zettabytes during 2012. This is 48% up from 2011.[11]
- Mark Liberman calculated the storage requirements for all human speech ever spoken at 42 zettabytes if digitized as 16 kHz 16-bit audio. This was done in response to a popular expression that states “all words ever spoken by human beings” could be stored in approximately 5 exabytes of data (see exabyte for details). Liberman did “freely confess that maybe the authors [of the exabyte estimate] were thinking about text.”[12]
- Research from the University of Southern California reports that in 2007, humankind successfully sent 1.9 zettabytes of information through broadcast technology such as televisions and GPS.[13]
- Research from the University of California, San Diego reports that in 2008, Americans consumed 3.6 zettabytes of information.
- Internet Traffic to Reach 1.3 Zettabytes by 2016
Yottabyte (1 000 000 000 000 000 000 000 000 Bytes)
These computers, like IBM Watson can process 500 gigabytes/second, the equivalent of a million books, of structured and unstructured data.
Overtime, we will start to see more companies leveraging these cogitative computers and licensing them to all of us, the healthcare professional at hospitals around the country.
How can it help?
1. enable information gathering and expertise sharing, which can help teams collaborate and design programs that support patient care and program success.
2. ability to see and analyze once-invisible data and evaluate it based on the leading medical literature, the most current medical information, and evidence-based guidelines. The insights will help as you plan care for each patient’s specific health needs.
3. insurers can proactively manage preventive care for large numbers of people with the goal of avoiding unnecessary utilization
4. able to sift through available clinical trials and ensure that more patients are accurately and consistently matched to the right trials
5. see more data and deliver more insights so you can create a more informed program that keeps your patients moving toward their goals to better health.
16. Genomics Editing/Splicing
Genome editing is a way of making specific changes to the DNA of a cell or organism. An enzyme cuts the DNA at a specific sequence, and when this is repaired by the cell a change or ‘edit’ is made to the sequence.
Intellia is a leading genome editing company, focused on the development of proprietary, potentially curative therapeutics using a recently developed biological tool known as the CRISPR/Cas9 system. Intellia believes the CRISPR/Cas9 technology has the potential to transform medicine by permanently editing disease-associated genes in the human body with a single treatment course.
17. Bioabsorbable Stents
Every year, 600,000 people have metal coronary stents put into their chests to treat coronary artery blockage. Most of the time, that stent stays there forever, long after its mission is complete. The stents may inhibit natural blood flow and cause other complications, like blood clots.
What if they could just disappear? That is a long-sought goal researchers have finally met. This past July, the first bioabsorbable stent was approved in the United States. Made of a naturally dissolving polymer, the stent widens the clogged artery for two years before it is absorbed into the body in a manner similar to dissolvable sutures.
The disappearing stent leaves behind a healthy natural artery. The patient is free to go off blood clotting medication and qualifies for a broader range of medical treatments. What’s more, recovery time has been reduced from three to four weeks for metal stents to just a few days.
Only one version of an absorbable stent has been FDA approved but more are coming. Experts believe the market potential will approach $2 billion in six years. While the full impact of vanishing stents is yet to be seen, 2017 is the year the technology becomes a game changer.